Simple concept about Bohr's atomic model
Here is very simple concept about Bohr's atomic model:
Well, Bohr's atomic model talks about what happens when an electron revolves round a nucleus.
Bohr's atomic model says that when an electron revovles round a nucleus it revolves in an certain orbit having defined energy. There are many orbits surrounding the nucleus. the nearest orbit has the lowest energy and the farthest one has the the highest enegy. That means electron can't revolve in random order otherwise they may collide and along with this Bohr's atomic model also says that electron has certain amount of energy stored in it.
Bohr's atomic model terms that electrons can only revolve in those orbits where the energy of the electron and the energy of the orbit gets matched. Bohr's atomic model says that t he energy of these orbits are multiples of (see the image)
he energy of these orbits are multiples of (see the image) .Now an electron can't move in a definate orbit all the time, while moving it either gains energy or looses energy. Bohr's atomic model assures that when an electron looses energy it jumps to a lower orbit and when it gains energy it jumps to a higher one.
.Now an electron can't move in a definate orbit all the time, while moving it either gains energy or looses energy. Bohr's atomic model assures that when an electron looses energy it jumps to a lower orbit and when it gains energy it jumps to a higher one.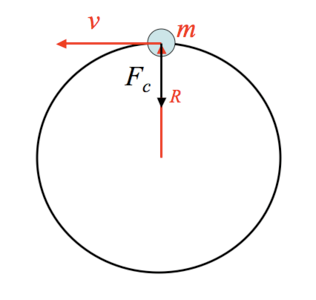
This is the major concept.
Bohr's atomic model gives the value of the velocity of the electron that moves around it.
As the electron moves in circular path it needs a force which keeps it inside the orbit. This force is Centripetal force.
So how does the electron gets centripetal force, it is the coulomb force that provides the necessary centripetal force.
Now coulomb's force is the required centripetal force, both must be equal then.

Bohr's atomic model also gives the relation that the angular momentum of the electron moving in an orbit is equal to nth times the plancks constant where n refers to the number of orbit.
moving in an orbit is equal to nth times the plancks constant where n refers to the number of orbit.





Well, Bohr's atomic model talks about what happens when an electron revolves round a nucleus.
Bohr's atomic model says that when an electron revovles round a nucleus it revolves in an certain orbit having defined energy. There are many orbits surrounding the nucleus. the nearest orbit has the lowest energy and the farthest one has the the highest enegy. That means electron can't revolve in random order otherwise they may collide and along with this Bohr's atomic model also says that electron has certain amount of energy stored in it.
Bohr's atomic model terms that electrons can only revolve in those orbits where the energy of the electron and the energy of the orbit gets matched. Bohr's atomic model says that t
 he energy of these orbits are multiples of (see the image)
he energy of these orbits are multiples of (see the image) .Now an electron can't move in a definate orbit all the time, while moving it either gains energy or looses energy. Bohr's atomic model assures that when an electron looses energy it jumps to a lower orbit and when it gains energy it jumps to a higher one.
.Now an electron can't move in a definate orbit all the time, while moving it either gains energy or looses energy. Bohr's atomic model assures that when an electron looses energy it jumps to a lower orbit and when it gains energy it jumps to a higher one.
This is the major concept.
Bohr's atomic model gives the value of the velocity of the electron that moves around it.
As the electron moves in circular path it needs a force which keeps it inside the orbit. This force is Centripetal force.
So how does the electron gets centripetal force, it is the coulomb force that provides the necessary centripetal force.
Now coulomb's force is the required centripetal force, both must be equal then.


Bohr's atomic model also gives the relation that the angular momentum of the electron
 moving in an orbit is equal to nth times the plancks constant where n refers to the number of orbit.
moving in an orbit is equal to nth times the plancks constant where n refers to the number of orbit.





0 comments:
Post a Comment